
| (prev) | (top) | (next) |
Given that such electromagnetic fields
propagate energy in the direction of E ⊗ B,
we examine an instant
when E is along the y axis. For propagation along the x axis, B will be along the z axis.
Applying Faraday's law to a loop of height dy and width dx normal to B:
we have
Electromagnetic waves also undergo Doppler shifting, as shown here:
This implies that the electromagnetic field exerts a pressure
Electromagnetic Waves
∫closed loop B ⋅ ds = μ (I + ε dΦE/dt).
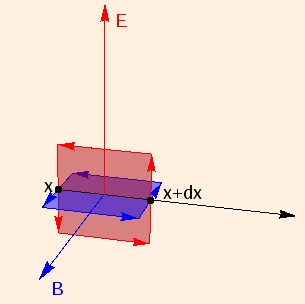
E(x + dx) * dy - E(x) * dy = - d(dx * dy * B)/dt
which in the limit dx → 0 becomes
dE/dx = - dB/dt.
Applying Ampere's law (with I = 0) to a loop of height dz and width dx normal to E gives us
B(x) * dz - B(x + dx) * dz = μ ε d(dx * dz * E)/dt
which in the same limit becomes
dB/dx = - μ ε dE/dt.
Differentiating the first equation with respect to x and the second with respect to t and equating the results gives us the electromagnetic
wave equation for E:
d2E/dx2 = d2E/dt2 / c2
with propagation velocity
c = 1 / √(μ ε) = 2.998 * 108 m/s = the speed of light.
Doing the reverse gives us the (same) wave equation for B.
E / B = ω / k = c.
radiation λ ν (Hz) energy (eV) source radio > 1 m < 3 * 108 < 1.24 * 10-6 low-energy atomic or molecular motions microwave > .1 mm < 3 * 1012 < .0124 rigid molecular motions infrared > 7000 Angstroms < 4.3 * 1014 < 1.78 molecular bond motions visible light > 4000 Angstroms < 7.5 * 1014 < 3.1 atomic electron transitions ultraviolet > 50 Angstroms < 6 * 1016 < 248 atomic electron transitions x-rays > .03 Angstroms < 1020 < 414 K electron transitions in heavy atoms gamma rays < .03 Angstroms > 1020 > 414 K nuclear decays
S = E ⊗ B / μ,
with units of W / m2. Since the integral of cos2 over one wavelength is 1/2, the intensity is
<S> = E2 / (2 μ c)
with u equal to the sum of the electric and magnetic energy densities:
= c <u>
ε E2 / 2 + B2 / (2 μ) = E2 / (2 μ c2) + (E / c)2 / (2 μ)
("<>" denotes the average over one wavelength, which introduces the factor of 1/2).
= E2 / (μ c2)
P = S / c
(under conditions of perfect absorption). Pressure is measured in Pascals: 1 Pa ≡ 1 N / m2.
Note that the units of pressure are identical to those of energy density.
P = 2 S / c.
| (prev) | (top) | (next) |
©2012, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.