
| (prev) | (top) | (next) |
These constants can be combined to set natural scales for our fundamental units:
It is also interesting to note that these constants can be combined in easy ways so as to "convert" one fundamental unit into any other; in
General Relativity (a geometric theory), it is usually convenient to express everything in terms of length, so that
This suggests that if indeed there is a theory which encompasses all physical phenomena (at least at the smallest scales), that all of
the fundamental physics quantities (length, time, mass and charge) will be interrelated.
Quantum Principles
E = h ν,
where h is Planck's constant = 6.626 * 10-34 J s.
I(λ,T) = 2 π h c2 / (λ5 (eh c / (λ kB T) - 1))
where kB is Boltzmann's constant = 1.381 * 10-23 J / K:
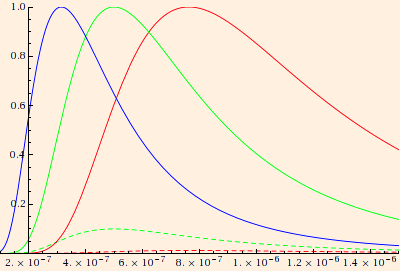
(The solid normalized curves correspond to the surface temperatures of Sirius A (9200 K), Sol (5800 K) and Betelgeuse (3800 K).
The dashed curves are proportional intensities for Sol and Betelgeuse.)
For a given temperature, the maximum is located at (2.9 * 10-3 m K) / T (Wien's law).
p = h / λ.
If φ energy is required to eject an electron, a potential ΔVs can be applied which will stop the process:
e ΔVs = h ν - φ
where ΔVs is the stopping potential and φ is the
work function. The frequency corresponding to zero stopping potential is νcutoff: the minimum
frequency required to eject an electron. Note that this relationship is independent of intensity.
Δx Δpx ≥ h / (4 π)
and
ΔE Δt ≥ h / (4 π)
Think in terms of the wavelength required to resolve a particle, and the resulting recoil.
(Some sources define these quantities in terms of h / (2 π) instead of h, or 4 π ε0 instead of ε0,
but the orders of magnitude are essentially unchanged.)
LP and tP are often interpreted as the smallest measurable intervals of distance and time; mP is the mass
of 5.9989 * 1022 electrons, and qP is the charge on 8.27756 electrons (as defined here).
| (prev) | (top) | (next) |
©2011, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.