
| (prev) | (top) | (next) |
where Z is the nuclear charge, the Bohr radius
<r> is the expected value (the most "probable value"), and is computed using
Also note that as decaying exponentials, they only approach zero asymptotically, but they have significant nonzero portions far
beyond the orbital radii suggested by Bohr's model.
Finally, note that for a hydride ion (H-), the electron wave functions overlap.
Because of charge screening of the nuclear charge by intervening electrons, electrons in an energy level n>1 of multielectron atoms
see an effective nuclear charge of approximately n.
The Atom
m v r = n h / (2 π).
Equating the centripetal force
to the Coulomb force (and E = K + U) gives us a corresponding discrete spectrum of orbital radii and energy levels:
a0 = ε h2 / (π m e2)
and the Rydberg energy
= .529 * 10-10 m (= .529 Angstroms)
E0 = m e4 / (8 ε2 h2)
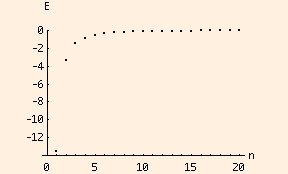
These are the first 20 energy levels of a Hydrogen atom:
= 13.606 electron Volts (1 eV = e J)
P ψ = - i (h / (2 π)) dψ/dx,
and the energy operator is
E ψ = i (h / (2 π)) dψ/dt,
where ψ(x, t) is
the electron wave function.
With these prescriptions, the statement of conservation of energy becomes Schrodinger's equation:
E ψ = P2 ψ / (2 m) + U(x) ψ
or
i (h / (2 π)) dψ/dt = - (h / (2 π))2 / (2 m) ∇2 ψ + U(x) ψ
Here ∇2 ψ ≡ ∇ ⋅ (∇ ψ). In general, solving this differential equation is beyond our
present means. But if we restrict ourselves to spherically symmetric wave functions, where now
∇2 ψ = d(r2 dψ/dr)/dr / r2,
and take ψ(x, t) = ψ(r) χ(t), with the Coulomb potential energy Schrodinger's equation becomes
i (h / (2 π)) dχ(t)/dt / χ(t) = - ((h / (2 π))2 (2 dψ/dr + r d2ψ/dr2) / (2 m r) +
(- e2 / (4 π ε r)) ψ) / ψ
This equation is obviously separable, and so both sides equal a constant (E). The solution for χ is
χ(t) = e-i E t / (h / (2 π))
and the right hand side is equivalent to the Laguerre equation. In general, it too is beyond our capabilities, but
the first few solutions are instructive:
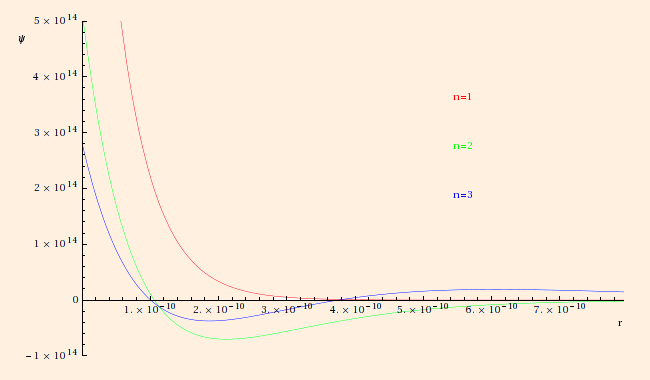
These wave functions have been normalized such that ∫all space ψ2 dx3 = 1.
Note the agreement with our previous analysis based on Bohr's quantization condition. While the expectation values for r are not the same, if
we substitute 3 a0 / 2 for a0 in Bohr's results, they still obey the relation
<r> = ∫ ψ2 r dx3,
which in our case is
4 π ∫0∞ ψ2 r3 dr.
If we interpret <r> as the most probable value, ψ takes the role of a probability amplitude,
and ψ2 (which in general is ψ* ψ, since ψ can be complex) takes the role of a
probability distribution.
rn = n2 a0 / Z
Note too that the second and third solutions have
nodes at r = 2 a0 and r = 3 (3 +- √ 3) a0 / 2, respectively. These nodes are reminiscent of the nodes in
standing waves, and lead to a similar interpretation for the electron wave functions.
The spin is an intrinsic angular momentum which creates a
magnetic moment, but is not associated with mechanical motion.
This applet will draw three dimensional plots of several complete (not simply spherically
symmetric) electron wave functions for Hydrogen atoms and Hydride ions.
These quantum numbers explain the form of the periodic table.
| (prev) | (top) | (next) |
©2012, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.