
| (prev) | (top) | (next) |
Note that since Celsius and Kelvin temperatures differ by an additive constant, a difference in temperatures ΔT is numerically the same in either unit.
Q = m c ΔT = C ΔT,where c is the specific heat of the substance and C is the heat capacity of the object.
When the volume is constant, we often write
ΔQ = n cv ΔTwhere n is the number of moles and cv is the molar specific heat at constant volume.
Likewise, when the pressure is constant, we often write
ΔQ = n cp ΔT,where cp is the molar specific heat at constant pressure.
Q = m L,where L is the latent heat (Lf of fusion or Lv of vaporization).
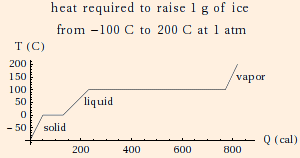
| c(water) = 1 cal/gK (1 cal (calorie) = 4.186 J) | Lf(water) = 80 cal/g |
| c(ice) = 0.51 cal/gK | Lv(water) = 540 cal/g |
| c(water vapor) = 0.48 cal/gK (at constant pressure) |
dQ/dt = k A dT/dx,where k is the thermal conductivity, A is the cross-sectional area through which heat is being transferred and dT/dx is the temperature gradient. This expression is isomorphic to Ohm's law with the substitutions:
dQ/dt = σ A e T4,where σ is the Stefan-Boltzmann constant (5.67 * 10-8 W / (m2 K4)) and e is the emissivity, equal to the fraction of incoming radiation which is absorbed. An emissivity of 1 corresponds to a black body.
This result follows from integrating the black body distribution from λ = 0 to ∞. That integral is the power per unit area, and e < 1 if the source is not a black body. The Stefan-Boltzmann constant is the coefficient of T4 in the integral, equal to2 kB4 π5 / (15 c2 h3).
| (prev) | (top) | (next) |
©2012, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.