
| (prev) | (top) | (next) |
(Here we used ∫0∞ x2 e-a x2 dx = (√ π) / (4 a3/2).)
= m N <v2> / (3 V)
In general, the Equipartition theorem tells us that
each quadratic degree of freedom in <E> contributes k T / 2.
= χ R / 2
= - (γ - 1) P dV
The polytropic equation of state is
Statistical Mechanics
f(E) ~ e-E / (k T).
If all of the energy is kinetic, and the gas is uniform, f(E) = e-m v2 / (2 k T) = f(v).
Note: this assumes that the particles in the gas are distinguishable. If they are not, the distribution function depends on the
spin of the particles. For particles of integer spin, we have the Bose-Einstein distribution
f(E) ~ 1 / (A eE / (k T) - 1),
and for particles of half-integer spin, we have the Fermi-Dirac distribution
f(E) ~ 1 / (A eE / (k T) + 1).
Note that this is a normal distribution with standard deviation √ (k T / m).
For air at room temperature (with molecular weight 28.94 g, temperature 20 C and mean speed 463 m/s), σ = 290.244 m/s:
In three dimensions we normalize this with respect to position and velocity as
∫ f(v) d3r d3v = N,
where N is the total number of particles in the gas. Now d3r = dV; assuming that the velocity distribution is
isotropic (does not depend on direction, i.e., is spherically symmetric), we have
d3v = 4 π v2 dv.
Integrating with respect to v from 0 to ∞, we find
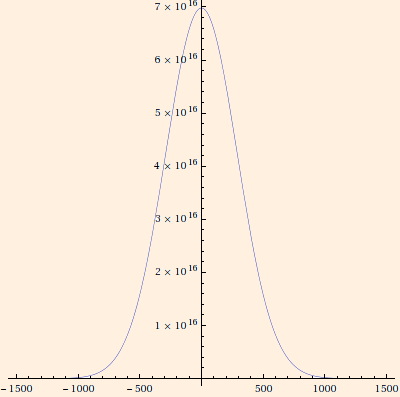
f(v) = (N / V) (m / (2 π k T))3/2 e-m v2 / (2 k T).
The graph above was plotted using this normalization and standard pressure, for a mole of air occupying 22.4L. The right side of the plot
is the relevant part, considering we are now treating v as a speed. For the 3-dimensional isotropic normal distribution, almost 87% of the molecules
will have speeds within one standard deviation of the mean.
<g(v)> = (V / N) ∫ g(v) f(v) d3v.
P = Fz / A = dpz/dt / A
= (1 / A) * the number of particles colliding per unit time * average momentum transfer
<P> = (1 / A) ∫ f(v) * (A dz / 2) / dt * 2 m vz d3v
(V = A dz, and half of the particles are moving away from the wall during the time dt)
= m ∫ f(v) vz2 d3v
(<vz2> = <v2>/3 since v is isotropic)
= 2 N <E> / (3 V)
P V = n R T,
where P is the pressure, V is the volume, n is the number of moles, and T is the temperature.
R = NA kB is the molar gas constant, equal to 8.314 J / (mol K).
Equations of state are usually thought of as relating pressure and density; in those terms, the ideal gas equation of state is
P = (R T / (molecular weight)) ρ.
dE = dQ + dW
where dE is the change in energy of the system, dQ is the heat added to it and dW is the work done on it:
W = -∫ P dV
cv = dQ/dT / n = dE/dT / n
and
(constant volume implies that W = 0)
= d(χ N k T / 2)/dT / n
cp = dQ/dT / n = (dE/dT + P dV/dT) / n
χ is the sum of translational, rotational and vibrational modes. All atoms and molecules possess 3 translational degrees of freedom; linear
molecules have 2 more rotational modes, whereas nonlinear molecules have 3 more rotational modes. Vibrational degrees of freedom require
sufficient energy to rise to an excited quantum state. For gases, room temperature is mostly insufficient to the task.
Note that vibrational modes have both a kinetic and a potential degree of freedom.
= cv + R.
(since P is constant, P dV/dT = d(P V)/dT = n R )
dE = n cv dT = - P dV.
Since
d (P V) = P dV + dP V = n R dT
we have
= - (R / cv) P dV
dP V = - γ P dV.
Integrating we find
P Vγ = constant.
Note that for an ideal gas, γ = 1 + R / cv = 1 + 2 / χ.
(P + α β / ν2) (ν - β) = R T,
where α is the interaction energy in Joules per mole, ν is the molar volume of the gas, and
β is NA times the effective volume of a single molecule (which is approximately 4 times the actual volume,
since the center of another molecule cannot be closer than 2 molecular radii away). Notice that
in the limit of non-interacting point-like molecules, the Van der Waals equation of state reduces to that of an ideal gas.
dQ/dT = n * a constant (c)
The polytropic index
κ = (cv - c) / (cp - cv)
has useful values
P ~ ρ1 + 1/κ.
P = w ρ
and
| (prev) | (top) | (next) |
©2011, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.