
| (prev) | (top) | (next) |
(Throughout, we will assume the processes involve an ideal gas with constant n.)
In a cyclic process, the state variables all return to their initial values after one cycle.
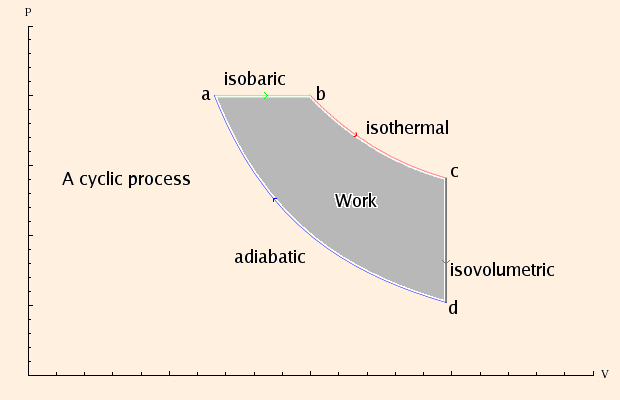
Assuming this PV diagram describes a process involving an ideal gas, we can deduce the following:
| as a→b | Pa = Pb | ΔEa→b = n cv (Tb - Ta) | ΔQa→b = n cp (Tb - Ta) | Wa→b = -Pa (Vb - Va) |
| at b | Tb = Pb Vb / (n R) | Tb > Ta | ΔQa→b > 0 | Wa→b < 0 |
| as b→c | Tb = Tc | ΔEb→c = 0 | ΔQb→c = -Wb→c | Wb→c = -n R Tb ln (Vc / Vb) |
| at c | Tc = Pc Vc / (n R) | ΔQb→c > 0 | Wb→c < 0 | |
| as c→d | Vc = Vd | ΔEc→d = n cv (Td - Tc) | ΔQc→d = n cv (Td - Tc) | Wc→d = 0 |
| at d | Td = Pd Vd / (n R) | Td < Tc | ΔQc→d < 0 | |
| as d→a | Pd Vdcp/cv = Pa Vacp/cv | ΔEd→a = n cv (Ta - Td) | ΔQd→a = 0 | Wd→a = n cv (Ta - Td) |
| at a | Ta = Pa Va / (n R) | Ta > Td | Wd→a > 0 |
In addition, we know that ΔEa→b + ΔEc→d + ΔEd→a = 0, but in this case it only tells us that Tb = Tc.
From this we can compute the total heat added during the cycle:
n cp (Tb - Ta) + n R Tb ln (Vc / Vb)and the total work done by the gas:
Pa (Vb - Va) + n R Tb ln (Vc / Vb) - n cv (Ta - Td),corresponding to the shaded area in the PV diagram.
Note that heat enters this "engine" in steps a→b and b→c, and exits it in step c→d; the total work done by the engine is positive. If the cycle were operated in reverse, it would be a "refrigerator", taking heat from a cooler environment during step d→c and exhausting it to a warmer environment from c→a; the total work done by the refrigerator is negative. The meaning of this is that it requires external work to move heat from a cooler to a warmer place.

Suppose we are given Pa = 200,000 Pa, Pb = 300,000 Pa, Pc = 100,000 Pa and Va = 65 L.
We know that Pd = Pc, Vb = Va, and Tb = Tc. We can use the ideal gas law to compute the temperatures at points a and b from the pressure and volumes at those points: 390.907 K and 586.36 K, respectively. From the latter we can compute the volume at point c, which is 195 L.
We can use the adiabatic equation for an ideal gas:
Pd Vdγ = Pa Vaγ,to compute Vd = 98.5216 L. Finally, we can use the ideal gas law to find that Td = 296.252 K.
Since b to c is an isothermal expansion, the first law tells us that ΔQb→c = - ΔWb→c. Using the ideal gas law, we find
ΔWb→c = - ∫bc P dVUsing the equations for isovolumetric and isobaric processes, we have= - n R Tb ∫bc dV / V(Note the sign: the gas does external work.)= - n R Tb ln (Vc / Vb) = -21,422.9 J.
Finally, consistent with the fact that ΔE = 0 for a cycle, we compute
ΔQa→b = n cv (Tb - Ta) ΔQb→c = n R Tb ln (Vc / Vb) ΔQc→d = n cp (Td - Tc) ΔQd→a = 0 = 9750 J = 21,422.9 J =-24,119.6 J
ΔWa→b = 0 ΔWb→c = - n R Tb ln (Vc / Vb) ΔWc→d = Pc (Vc - Vd) ΔWd→a = cv (Pa Va - Pd Vd) / R = -21,422.9 J = 9647.84 J = 4721.76 J
ε = W / QhIn the example above, Qh = 31,172.9 J, W = 7053.33 J, Qc = 24,119.6 J and ε = 22.6265%.= (Qh - Qc) / QhHere, energy is taken in as Qh, work W is done by the engine, and the waste heat is discharged as Qc.= 1 - Qc / Qh.
This calculation obscures some interesting facts:For an air conditioner, heat pump in air conditioning mode, or refrigerator, ε = Qtransferred / Wrequired. The efficiency of a refrigerator can be greater than 1, since
- for this cycle, as the number of degrees of freedom of the gas χ→∞ (with all other values unchanged), ε→0 as 1/χ (more heat must be removed during isobaric cooling to lower the temperature the same amount, because more of the heat comes from non-translational degrees of freedom which do not affect temperature);
- ε does not depend on n; increasing n will only lower the temperatures (via the ideal gas law; with more molecules, less energy is available per molecule);
- while the efficiency is a complicated function of the given values, it is turns out that, holding all other values constant, increasing Pb or decreasing Pc will increase ε, while the value of Va does not alter ε at all and the chosen value of Pa is almost optimal.
Of course, for another cycle, the results can be very different.
ε = Qc / (Qh - Qc)where Qc is the heat removed from the cooler environment and Qh is the heat exhausted into the warmer environment. Note that Qh must be greater than Qc.= 1 / (Qh / Qc - 1),
ΔS = - ΔQh / Th + ΔQc / Tc.In general, ΔS = ∫ dQ / T.
For the example above, ΔSb→c = 36.5355 J/K, and of course ΔSd→a = 0. To compute ΔSa→b and ΔSc→d, we use the ideal gas law to express T as a function of P and V:
ΔSa→b = ∫ n cv dT / Tand= ∫ n cv (V dP / (n R)) / (P V / (n R))= n cv ln (Pb / Pa) = 20.2262 J/K
ΔSc→d = ∫ n cp dT / TThe changes in entropy of the reservoirs are of course dependent on their temperatures. Assuming that the isobaric compression lost heat to a reservoir at room temperature (< Td), and that the isochoric and isothermal processes gained heat from a reservoir at 320 C (> Tb),= ∫ n cp (P dV / (n R)) / (P V / (n R))= n cp ln (Vd / Vc) = -56.7617 J/K
ΔScold reservoir = 24119.6 / 293.15 J/K = 82.277 J/K andso thatΔShot reservoir = -31172.9 / 593.15 J/K = -52.555 J/K,
ΔSreservoirs = 29.722 J/K,which is greater than zero, as required by the second law of thermodynamics.
If we did not know T as a function of P and V, it would be necessary to connect each pair of endpoints with a combination of adiabatic and isothermal processes, and compute the change in entropy using those new processes. For example:
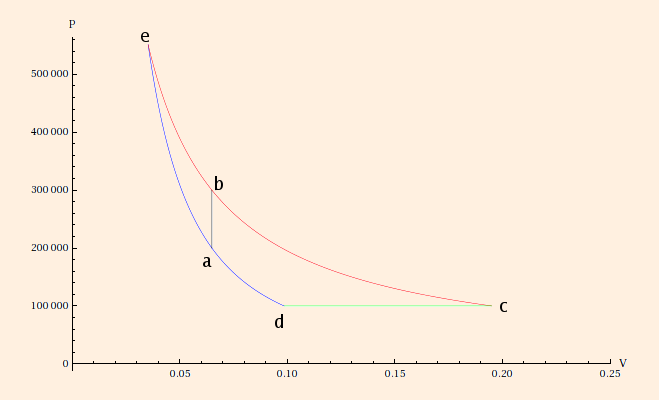
Process a→e is an adiabatic process, and e→b is isothermal. Pe = 551,135 Pa and Ve = 35.3815 L, from
Pa Vaγ = Pe VeγThis gives us (using the same equations as above)= n R Tb Veγ - 1
ΔQa→e = 0 andSince entropy is a state variable,ΔQe→b = 11,859.9 J.
ΔSa→b = ΔSe→b = 20.2262 J/K,as before. Either way, the entropy change of the engine is zero, because it is a cycle: all state variables, including entropy, end at the same state in which they began. Therefore ΔSc→d = -56.7617 J/K.
What makes it so is that each step of the Carnot cycle is reversible: all changes of state are infinitesimally close to equilibrium.
Reversible changes of state are infinitely long; all real changes of state are irreversible.Since it is reversible, the total entropy change of the Carnot cycle reservoirs is zero. This gives usIn isothermal processes, by definition the gas is in thermal equilibrium with the reservoir. An adiabatic process can be irreversible:
Consider a closed, thermally insulated volume with a partition in the middle, such that nearly all of the gas molecules are on one side of the partition; the other side is a nearly perfect vacuum. With removal of the partition, the gas adiabatically but irreversibly doubles in volume.A reversible adiabatic process would be a change in volume by infinitesimal steps while the gas is thermally insulated.
0 = - Qh / Th + Qc / Tcor
Qc / Qh = Tc / Th.If we attempt to compute the entropy change for c→d by finding a point f on the PV diagram such that c→f is adiabatic and f→d is isothermal, we find that we have constructed a Carnot cycle:
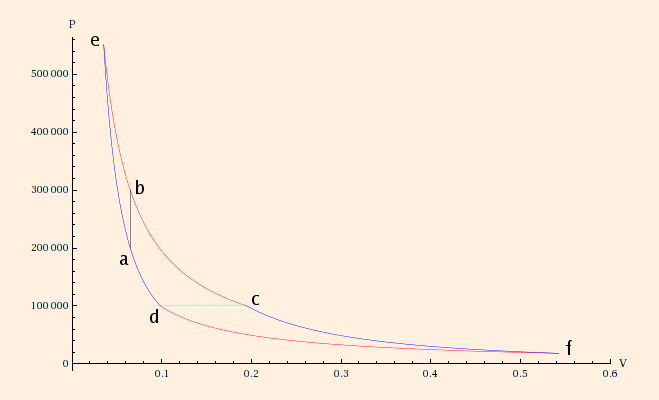
Hence the total entropy change is zero including the reservoirs, and its efficiency is
1 - Td / Tb = 49.4761%.The efficiency of a Carnot Refrigerator is Tc / (Th - Tc).
ε = 1 - (Td - Ta) / ((1 + 2 / χ) (Tc - Tb))
ε = 1 - (Vsmall / Vlarge)(2 / χ)
ε = 1 / (1 + Tlower / |Δ T| + χ / (2 ln (Vlarge / Vsmall)))
In each case, ε was computed for an ideal gas. Note that in each case, it is independent of n, and increases with decreasing χ. This generalizes what we saw above: if we think of work as the result of increased temperature, less heat input is required to change the temperature of a gas with fewer degrees of freedom.
| (prev) | (top) | (next) |
©2012, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.