
In the last section, it was noted that electrons in covalent bonds can absorb or emit photons just as electrons bound to a single atom can. In fact, every bond in a molecule acts like a tiny spring and can therefore oscillate along the axis of the bond:
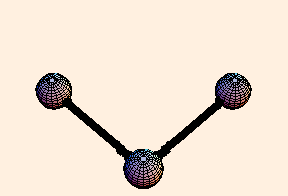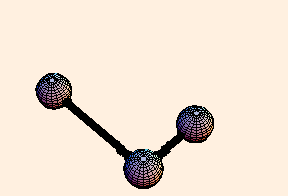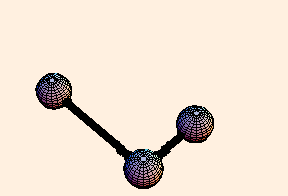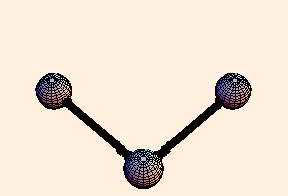
The spring constant for a single bond is typically around 6.5 eV / Angstrom2, while for a double bond it is twice that. For single bonds, the bond length oscillates on the order of a third of an Angstrom from its equilibrium position. For double bonds, not surprisingly, the oscillation is a little over half that.
For any pair of bonds in a molecule, there can also be an oscillation in the angle between them:
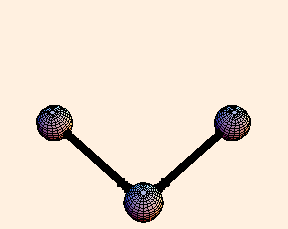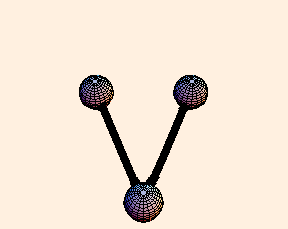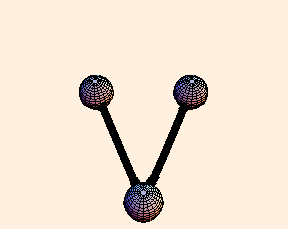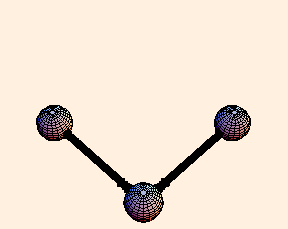
For this situation, we write the energy of the bond as
U = k (Δ θ)2 / 2.For tetrahedral bond angles, this spring constant is about 1 eV / radian2, while for trigonal bond angles it is around 1.5 eV / radian2. Typical angular displacement are on the order of half a radian.
Looking at these energies, we see that the associated photons are typically in the infrared range. There is a pattern developing here. If we further consider kinetic motion of whole molecules, we see that the wavelength of radiation scales as the size of the source: smaller sources emit (and absorb) higher energy photons, while larger sources emit (absorb) lower energy photons:
Microwave wavelengths correspond to whole molecule rotational modes. The microwave oven operates on the principle that there are a few rotational modes which are relatively easy to excite in water and fat molecules. Since these are the principle constituents of the foods we eat, the energy they absorb dissipates as heat (translational motion), and our food becomes hot. This is why water boils but the cup remains relatively cool, and why the cheese bubbles on the pizza before the crust is hot.
radiation λ ν (Hz) energy (eV) source radio > 1 m < 3 * 108 < 1.24 * 10-6 conduction electrons, low-energy atomic or molecular motions microwave > .1 mm < 3 * 1012 < .0124 rigid molecular motions infrared > 700 nm < 4.3 * 1014 < 1.78 molecular bond motions visible light > 400 nm < 7.5 * 1014 < 3.1 atomic electron transitions ultraviolet > 5 nm < 6 * 1016 < 248 atomic electron transitions x-rays > .03 Angstroms < 1020 < 414 K electron transitions in heavy atoms
Now it is time to compute the characteristics of photons which excite the modes pictured above.
It is often suggested that the full Moon has an effect on the psychological condition of certain mammals.
In fact, there does seem to be a correlation (although not necessarily a causal relationship) between the phase of the Moon
and the frequency of emergency room cases in hospitals. The Moon is full when the Earth is between the Moon and the Sun, and it is a "new"
Moon when it is between the Sun and the Earth. The average distance between the centers of the Earth and the Moon is 3.84 * 108 m,
and the radius of the Earth is 6.378 * 106 m. The mass of the Moon is 7.36 * 1022 kg.
We can compute the difference in the gravitational potential energy
of electrons and molecules in your body (assume an average molecular mass of 2.453 * 10-26 kg for human tissue) between new and full Moon
(ignoring the Earth). Remember that you would be standing on the night side of the Earth; assume that you are colinear with the two positions of the Moon.
The energy difference is about 6.5 * 10-5 eV, which means that the effect should involve only rigid molecular motions.
The corresponding frequency is about 16 GHz, only a few times higher than many cell phones use. Does the Moon deserve the blame it often gets?
The next section describes nuclear processes.
©2013, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.