We saw in the last section that the energy of an atomic electron is proportional to the square of the ratio of two integers: the atomic number Z and the energy level n. This means that the energy is quantized: it can only have one of a discrete set of values. This is analogous to the situation we had with MRI, where the energy of the nuclear magnetic moment depended on the spin. Just as the nuclei could flip their spins with the absorption or emission of a photon of the correct energy, atomic electrons can change their energy levels via the same mechanism. If a photon has the same energy as the difference between two allowed energy levels, an electron at the lower level can absorb it and move to the higher level. If an electron moves to a lower level, it emits a photon with the correct frequency to conserve energy. When either of these things happen, we say that the electron has undergone a transition to another level. If an electron at energy level n absorbs a photon of energy -En, it is kicked out of the atom altogether. This means that -En is its ionization energy.
If we restrict ourselves to the naturally occurring elements, Z can be any integer from 1 (Hydrogen) to 92 (Uranium). When a given atom is in its ground state, all of its electrons are at their lowest possible energy level. The highest value of n for such an atom is 7 (in Uranium), but as we saw in the last section, an excited electron can have any value of n. Of course, as n approaches infinity, the energy differences between levels approaches zero, but there are still in any given atom a large number of significantly different energy levels. This means that there is a wide variety of photon energies which can cause transitions.
Photons associated with visible light have wavelengths in the range 700 (red) to 400 (blue) nm. Wavelengths above 700 are called infrared, and those below 400 are ultraviolet. Very high energy photons have wavelengths below 10 nm, and are called x-rays. All of these wavelengths are associated with atomic electron transitions, although it is not uncommon for the electrons in covalent bonds to absorb or emit photons in the visible and ultraviolet wavelengths.
It is important to note that atomic electrons can move to higher energy levels without photon absorption. Electrons can absorb energy in inelastic collisions with other atoms or molecules, subsequently losing that energy via incandescence: the glowing of hot atoms. Many, if not most, photons are created in this manner: in light bulbs, in fires and in stars. Electrons can also absorb energy from an external electric field, as occurs in fluorescent lights.
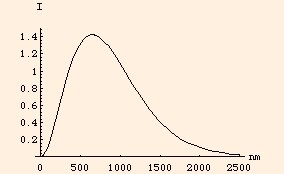
When a photon is absorbed by an atomic electron, the electron does not have to return to its original energy level when it emits another photon. This process is called inelastic scattering. An important example of this occurs in the sun: photons more energetic than x-rays are created in the core, but as they climb out of the sun's gravitational field they lose energy, so that the photons emitted from the surface are mostly in the visible wavelengths:
(the vertical scale is relative intensity). Note that even though we speak of a single photon being scattered, the photon which is emitted is not the same photon that was absorbed: the absorbed photon no longer exists, its energy and angular momentum having been transferred to the absorbing electron.
Of course, an absorbed photon does not have to be re-emitted: its energy can be used for chemical purposes. Photosynthesis and sight are two prime examples. When a photon hits your retina, it is absorbed and the energy is used to start the process of image formation. Absorption of photons by chlorophyll results in the production of molecules used by the plant to store energy. And in the process of fluorescence, photons are absorbed and stored for a relatively long period of time before being re-emitted. During that time, we say the the electron is in a meta-stable state: it is not at its lowest energy, but its relaxation time is relatively long.
Photons can also interact with electrons without losing energy, similar to an elastic collision: this is of course elastic scattering. It is the process responsible for the blue daylight sky: the scattering is inversely proportional to the fourth power of the wavelength, so blue photons are scattered much more than any other color and the sky appears to be a blue solid object.
A visible object's color is determined by the frequencies which are not absorbed by its atomic electrons. Chalk is white because it absorbs primarily in the infrared and ultraviolet: visible light is almost completely reflected. Copper compounds are blue-green because they absorb red photons and reflect most others. The exact frequencies are often shifted due to the surrounding atoms.
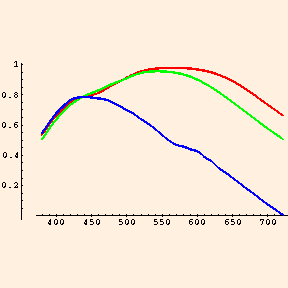
The cone cells of the human eye are sensitive to 3 wavelength ranges which the eye interprets as blue (narrow, with a peak near 419 nm), green (broader, with a peak near 531 nm) and red (also broad, with a peak near 558 nm, which is actually more like yellow!):
(from Vos, J. J. & Walraven, P. L.). All of the colors which your mind perceives are constructed from combinations of relative intensities of these three "wavelengths": red, green and blue are the only signals your brain receives from your eyes:
If 650 nm photons hit your retinae, your brain will receive a mixture of green and red signals, with more red than green but not too many of either. This will be interpreted as red. Similarly, 475 nm photons will cause about equal numbers of blue and green signals, with only a few red; this will be interpreted as a sort of bluish-green. The number of signals for any one of these ranges depends on both the intensity of the light and the sensitivity at that wavelength. This leads to a vaguely disturbing contrast between sensation and perception: your eye sends only three kinds of signals to your brain, yet your brain constructs the full color spectrum of reality from them. Now you can understand why people can have violent disagreements over what color something is: no two people see exactly the same thing, yet we assume that color is something objective.
Everything you see is the result of photons which have reflected off of the electrons in an object, only to be absorbed by the electrons in the cells on your retina. The world is literally alive with energy in the form of these photons. For example, a 40 W florescent light bulb (at 100 % efficiency) emits 40 J worth of photons each second. If we assume these photons have a wavelength of 550 nm (which is convenient as the "middle" of the visible spectrum), the energy in each photon is 3.612 * 10-19 J. This means that 1.11 * 1020 photons are emitted by the bulb every second!
The following applet will familiarize you with the characteristics of the photons involved in atomic electron transitions. Given the atomic number and the energy levels before and after the transition, you can compute the ionization energies, and the photon energy, frequency and wavelength.
The next section introduces us to molecular degrees of freedom and their associated quanta.
©2003, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.